Properties of matter & their measurement are divided in Physical and chemical properties.Every substance has unique or characteristic properties. These properties can be classified into two categories — physical properties, such as colour, odour, melting point, boiling point, density, etc., and chemical properties, like composition, combustibility, ractivity with acids and bases, etc. Physical properties can be measured or observed without changing the identity or the composition of the substance. The measurement or observation of chemical properties requires a chemical change to occur. Measurement of physical properties does not require occurance of a chemical change. The examples of chemical properties are characteristic reactions of different substances; these include acidity or basicity, combustibility, etc.
1) Measurement of physical properties
Quantitative measurement of properties is required for scientific investigation. Many properties of matter, such as length, area, volume, etc., are quantitative in nature. Any
quantitative observation or measurement is represented by a number followed by units
in which it is measured. For example, length of a room can be represented as 6 m; here, 6
is the number and m denotes metre, the unit in which the length is measured.
Earlier, two different systems of measurement, i.e., the English System and the Metric System were being used in different parts of the world. The metric system, which originated in France in late eighteenth century, was more convenient as it was based on the decimal system. Late, need of a common standard system was felt by the scientific community. Such a system was established in 1960 and is discussed in detail below.
1)-A]The International System of Units (SI)
The International System of Units (in French Le Systeme International d’Unités —
abbreviated as SI) was established by the 11th General Conference on Weights and Measures (CGPM from Conference Generale des Poids et Measures). The CGPM is an inter-governmental treaty organisation created by a diplomatic treaty known as
Metre Convention, which was signed in Paris in 1875.
Prefixes used in the SI system
1)-B] Mass and Weight
Mass of a substance is the amount of matter present in it, while weight is the force exerted
by gravity on an object. The mass of a substance is constant, whereas, its weight may vary from one place to another due to change in gravity. You should be careful in using these terms.
The mass of a substance can be determined accurately in the laboratory by using an
analytical balance Seen below fig.
The SI unit of mass is kilogram. However, its fraction named as gram (1 kg = 1000 g), is used in laboratories due to the smaller amounts of chemicals used in chemical reactions.
1)-C]Volume
Volume is the amount of space occupied by a substance. It has the units of (length)3. So in
SI system, volume has units of m3. But again, in chemistry laboratories, smaller volumes are used. Hence, volume is often denoted in cm3 or dm3 units.
A common unit, litre (L) which is not an SI unit, is used for measurement of volume of
liquids.
1 L = 1000 mL , 1000 cm3 = 1 dm3

Different units used to express volume
In the laboratory, the volume of liquids or solutions can be measured by graduated
cylinder, burette, pipette, etc. A volumetric flask is used to prepare a known volume of a
solution. These measuring devices are shown in Fig.

1)-D]Density
The two properties — mass and volume discussed above are related as follows:
Density= Mass/Volume
Density of a substance is its amount of mass per unit volume. So, SI units of density can be
obtained as follows:
SI unit of density = SI unit of mass /SI unit of volume
= kg /m3
This unit is quite large and a chemist often expresses density in g cm–3, where mass is
expressed in gram and volume is expressed in cm3. Density of a substance tells us about how closely its particles are packed. If density is more, it means particles are more closely
packed.
1)-E]Temperature
There are three common scales to measure temperature — °C (degree celsius), °F (degree
fahrenheit) and K (kelvin). Here, K is the SI unit. The thermometers based on these scales
are shown in Fig.
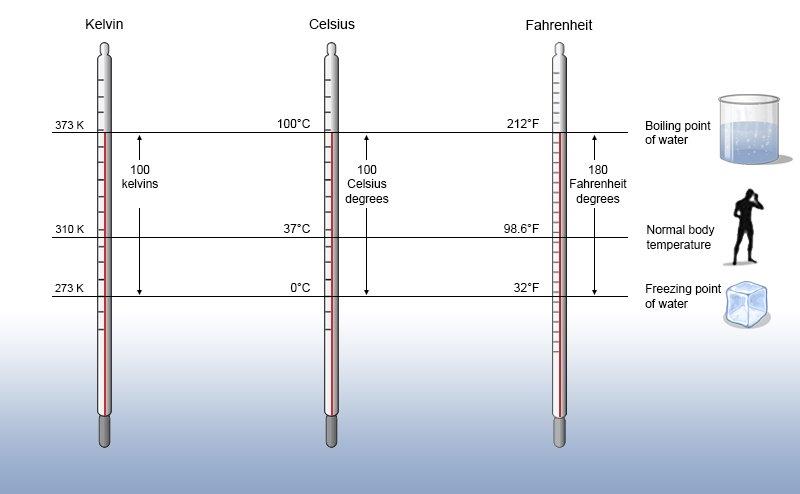
Generally, the thermometer with celsius scale are calibrated from 0° to 100°, where these two temperatures are the freezing point and the boiling point of water, respectively. The fahrenheit scale is represented between 32° to 212°.
The temperatures on two scales are related to each other by the following relationship:
°F = (°C) * 9/5 +32
The kelvin scale is related to celsius scale as follows:
K = °C + 273.15
It is interesting to note that temperature below 0 °C (i.e., negative values) are possible
in Celsius scale but in Kelvin scale, negative temperature is not possible.


Comments
Post a Comment
Please do not enter any spam link in the comment box